usp class vi vs iso 10993
ISO-10993 Standard USP Class VI Standard Other Industry Standards Page 2 Evaluation of Biocompatibility Page 6 Biomerics Polyurethane Resin Families Page 8 Quadrathane ALC Resin Results Page 9. Stickers 125 rs aprilia.
Adhesives Protective Materials And Equipment For Medical Device Assembly Intertronics
That said the lack of risk assessment in USP Class VI can be a problem.

. In 1995 the FDA adopted ISO 10993 as its biocompatibility approach. A rubber compound has set physical parameters it needs to meet. Sealable and weldable either pre- or post-sterilization c-flex 072 provides prolonged pump life sterilizable by gamma irradiation and autoclave product validation test summaries available upon request moldable bondable and formable for single-use assemblies and overmolds temperature range -67c to 135c -85f to 275f signifi.
However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not. Food Grade or USP Class IV Materials for Manufacturing Injectable Products. Because neither USP Class VI nor ISO 10993 are synonymous with biocompatibility testing asking for a.
Parylene has a long history of use as a protective coating for medical device biocompatibility and conforms to the USP Class VI and ISO 10993 standards. This is their current stance today. Inside Rubber Magazine Profiles The Rubber Group June 28 2022.
ISO-10993 is a standard that utilizes systemic toxicity and intracutaneous reactivity testing. In fact USP Class VI is sometimes seen as a minimum requirement for biocompatibility. Samsung gt- i9082 charging.
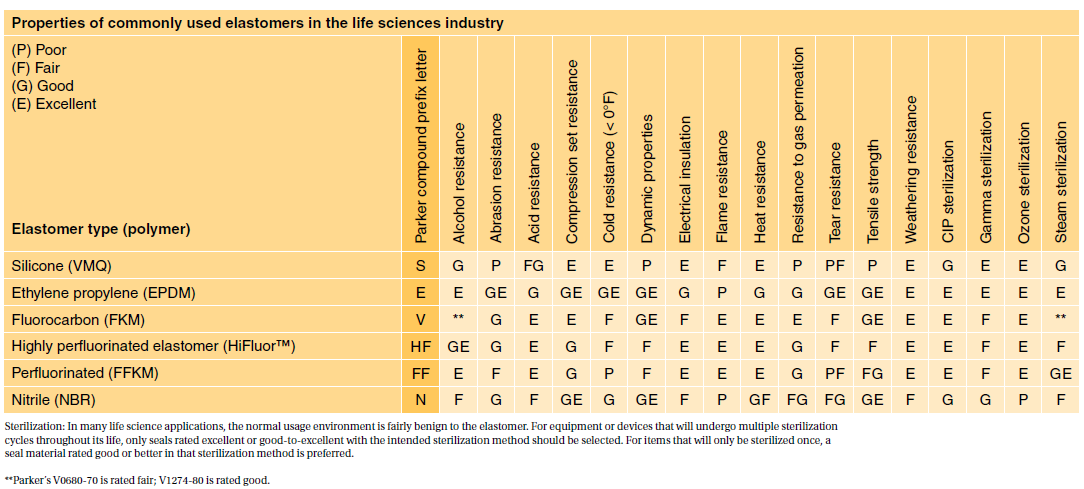
As an example usp class vi requires an intracutaneous irritation test which is also required for iso 10993 compliance. Most applications are fairly benign to elastomers. Iso 10993 is designed for medical products that remain permanently or for a very long time in the human body so for shorter applications a usp class vi or even a lower usp class certification is often sufficient.
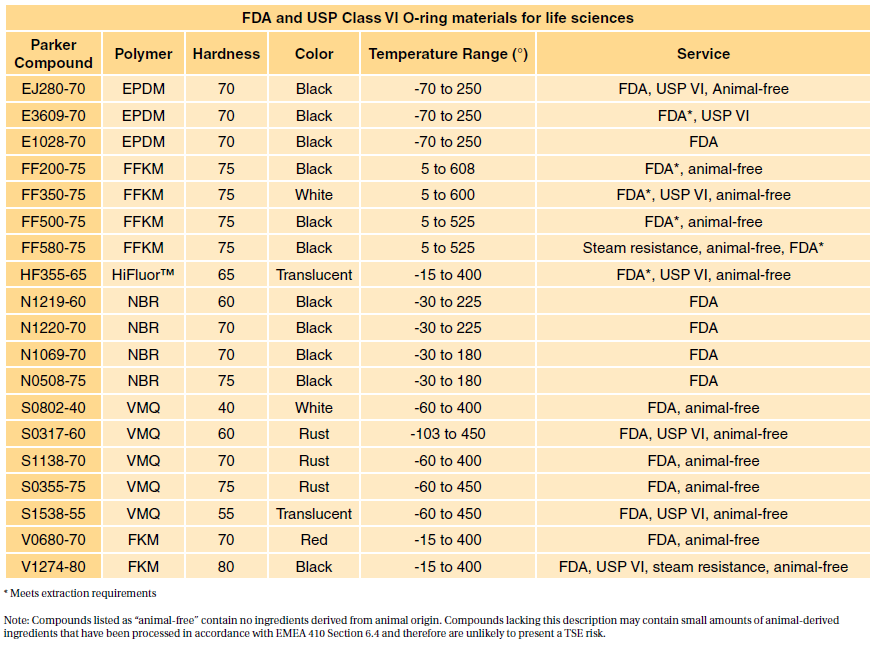
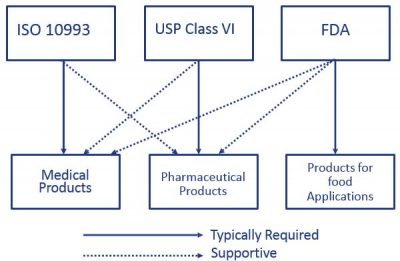
So does ISO 10993. Usp class vi and iso 10993. For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required.
Iso 10993 vs. Sumeet mixie jars in hyderabad. This post will take a deeper look at what biocompatibility is and how it is defined by the International Standards Organization.
The materials listed below are. It is transparent pin-hole free and conforms precisely to any surfaces features. Skf 6000 2z c3.
Class VI and ISO 10993 are recommendations for testing based on the use of the final device. How to Prevent Supply Chain Interruptions. Its possible that a USP Class VI material can also comply ISO 10993.
Biocompatibility - USP Class VI vs. Predictable Safe and Stable. A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing.
Ssc medical exam date. Tyre prices south africa. Statistical software in research methodology.
To begin let us address just what biocompatibility is. My understanding is that a statement in a 510 k that a material is usp class vi in general will not be accepted by fda as equivalent to evidence establishing that the nominally corresponding iso 10993 qualification is satisfied because the testing protocols are not identical and the test lab from which the results originally came presumably. A number of our plastic materials are ISO-10993 or USP Class VI capable.
1 Acute Systemic Toxicity 2 Intracutaneous Tocixity 3 Implantation Test If one is required to adhere to ISO 10933 then the only overlap between the two test methodsregimens is the Intracutaneous Toxicity test for an ISO Class A skin contact device. Steve Melito August 5 2020. The guidance memo wasis G95-1.
Other Medical Device Regulations World-Wide. Libros de hipnosis ericksoniana gratis. USP Class I II - Raw material supplier liability and responsibility.
Tripartite was the FDA biocompatibility approach until 1995. Mini off road teardrop trailer. Biocompatibility testing biocompatible materials biocompatible rubber ISO 10993 medical molder medical molding medical silicones USP Class VI.
ISO 134852016 - Medical Device Quality Management Systems. Under Tripartite USP class qualification was sometimes accepted as sufficient for lower-risk applications. Then you need to understand the differences between ISO 10993 and USP Class VI and the nature of each standard.
In fact USP Class VI has been largely superseded since the release of ISO 10993 in 1995. Suntour sf16 xcr32 coil- lo. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity.
Take an ASTM D2000 call out. Parylenes thickness is critically controlled and extremely consistent. Medical Molding and ISO 10993 series is intended for use by professionals appropriately qualified by training and experience who are able to interpret its requirements and judge the outcome of the evaluation for each medical device taking into consideration all the factors relevant to the medical device its.
USP Class VI typically requires the following tests. The Right Rheometer for Your Molded Rubber Parts March 30 2022. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI.
Medical Silicone Rubber Molding and Silicone Rubber Mold Materials. Unlike other rubber standards theres no one standard that engineers use for an approval. A more rigorous standard for the biological evaluation of medical devices is ISO-10993.
Typically the terms USP Class VI or ISO 10993 materials are used. USP Class VI demands an intracutaneous irritation test.

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing
Biocompatic Usp Class Vi Silicone Cable Alternative Northwire Inc
Material Selection Medical Injection Molding Xcentric Mold

Duraform Pa Certification Usp Class Vi Iso 10993 And Food Contact

Bal Seal Engineering Achieves Usp Class Vi And Iso 10993 5 Compliance For Medical Sealing Polymers Bal Seal Engineering

Usp Class Vi Foster Corporation
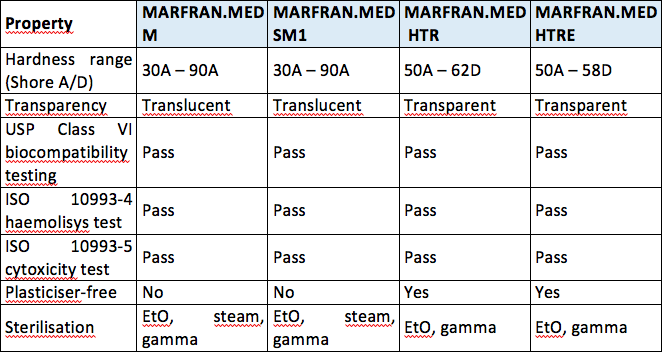
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News

Biocompatic Usp Class Vi Silicone Cable Alternative Northwire Inc

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Biocompatic Usp Class Vi Silicone Cable Alternative Northwire Inc